When a patient has had aggressive treatment for a brain tumor, another invasive procedure is one of the last things they want to go through. However, this is exactly what some patients being treated for glioblastoma brain cancer must endure.
Because of the aggressive nature of glioblastoma, patients need follow-up MRI scans every three to six months after treatment to check for recurrence. Unfortunately, the chemo-radiation used to treat glioblastoma can cause tissue changes that look like cancer on an MRI scan. Because of this, 20-30% of glioblastoma patients will require a highly invasive brain biopsy to find out if a suspicious tumor is cancer or not.
 Pallavi Tiwari, Ph.D., at the Case Comprehensive Cancer Center is working to address this treatment challenge by using machine learning and artificial intelligence to create computational maps of the brain that can help doctors tell the difference between tumor cells and benign radiation effects on MRI scans. These maps could not only help prevent unnecessary biopsies but are also revealing new details about the biology of these tumors.
Pallavi Tiwari, Ph.D., at the Case Comprehensive Cancer Center is working to address this treatment challenge by using machine learning and artificial intelligence to create computational maps of the brain that can help doctors tell the difference between tumor cells and benign radiation effects on MRI scans. These maps could not only help prevent unnecessary biopsies but are also revealing new details about the biology of these tumors.
“For the last 20 years or so, treatments for glioblastoma or the way these MRI images are evaluated have not changed,” said Tiwari. “Our computational techniques can extract information that is not visually appreciable to a radiologist to distinguish benign radiation effects from tumor recurrence using routine MRI scans.”
More than meets the eye
The researchers have previously shown that their machine learning techniques can distinguish radiation effects from tumor recurrence on MRI scans with 90% accuracy. With V Foundation funding, they are expanding their image analysis method to learn more about the tumors.
“When a biopsy is performed, the tissue comes from a single location, which may not be truly representative of what’s happening in the rest of the brain and the rest of the tumor,” said Tiwari. “Our machine learning approaches can capture the heterogeneity of a tumor and compare this among patients. Eventually, we hope to be able to detect certain molecular signatures that indicate who is most likely to respond to a specific type of treatment.”
The researchers have already found that glioblastoma tumors are not the same in men and women. They found that men tend to have molecular signatures associated with pathways that lead to more malignancy than those found in women, which may have important implications for treatment.
“This makes sense because we know that men are more likely to get this cancer and tend to have worse outcomes,” said Tiwari. “We also showed that the artificial intelligence computer models we’re creating are more accurate if they are built separately for men and women.”
Navigating the brain
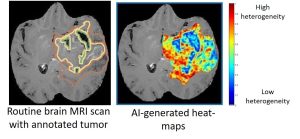 Using what they have learned so far, the researchers plan to conduct a pilot clinical trial that will use their imaging technology to help surgeons identify the best location to biopsy. Comparing the biopsy findings with their computational approach will demonstrate how well their technology is able to distinguish between regions of radiation effects and tumor recurrence.
Using what they have learned so far, the researchers plan to conduct a pilot clinical trial that will use their imaging technology to help surgeons identify the best location to biopsy. Comparing the biopsy findings with their computational approach will demonstrate how well their technology is able to distinguish between regions of radiation effects and tumor recurrence.
“We’ll create brain maps that the surgeon can use to navigate to the best location from which to take a biopsy,” said Tiwari. “This will be one of the first clinical trials to use machine learning for biopsy navigation in brain tumors.”
Tiwari says that obtaining funding for this type of pilot study can be difficult because it is a “high-risk, high-reward” project. If successful, it will demonstrate that the new method works well enough to be integrated in larger clinical trials while also giving clinicians more confidence in using machine learning and artificial intelligence approaches. Findings from the V Foundation project have also helped Tiwari secure an NIH grant that will support additional development of these tools.




